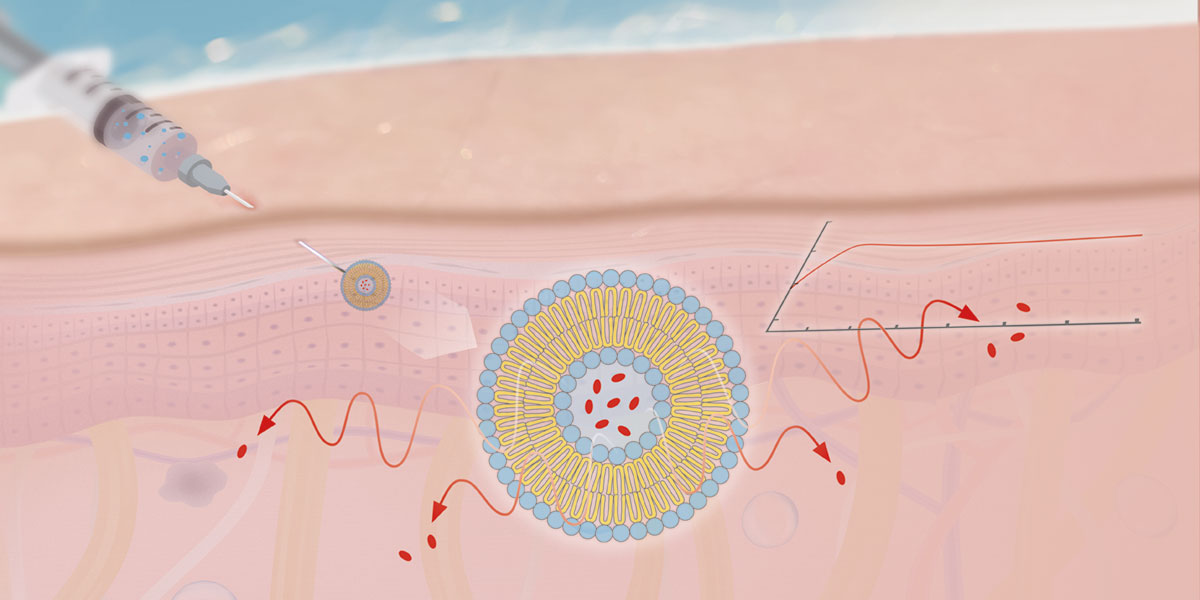
Because many drugs have a short biological half-life, multiple administrations are needed to achieve a therapeutic effect. In addition, drugs may not be suitable for oral administration because of degradation in the gastrointestinal tract and poor absorption. These include high molecular weight proteins and peptides. For such drugs, extended-release depot injectables for local or systemic applications enable a more patient-friendly, low frequency therapy, leading to a better compliance.
Extended drug release can be achieved by various formulation strategies. For intermediate release periods (days up to several weeks), lipid-based and especially phospholipid-based formulations are an interesting and innovative depot-injectable approach (Fig. 1).
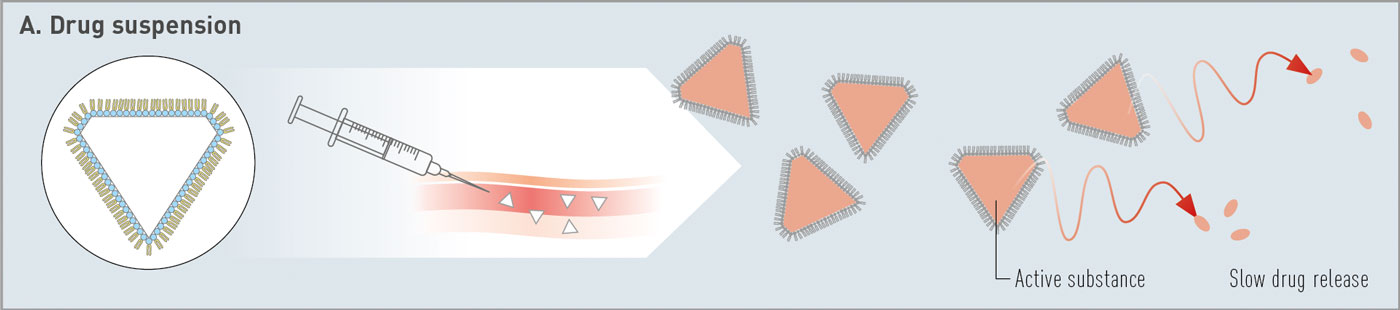
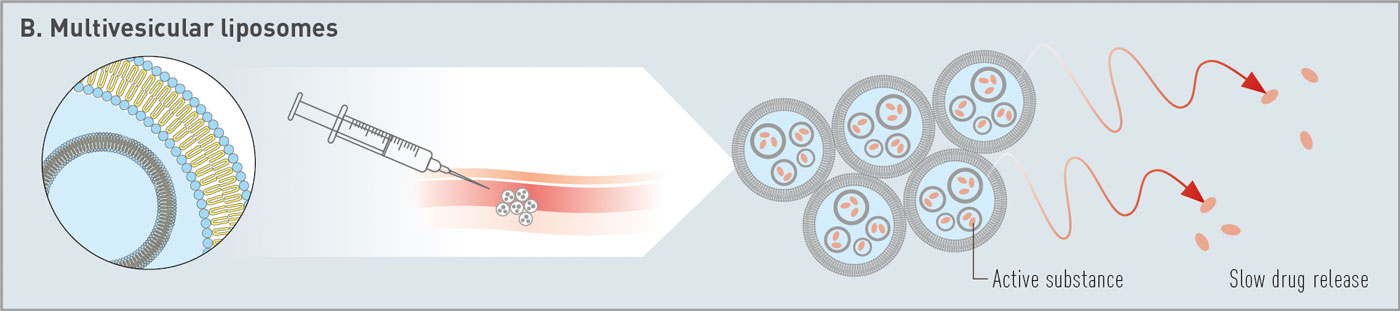
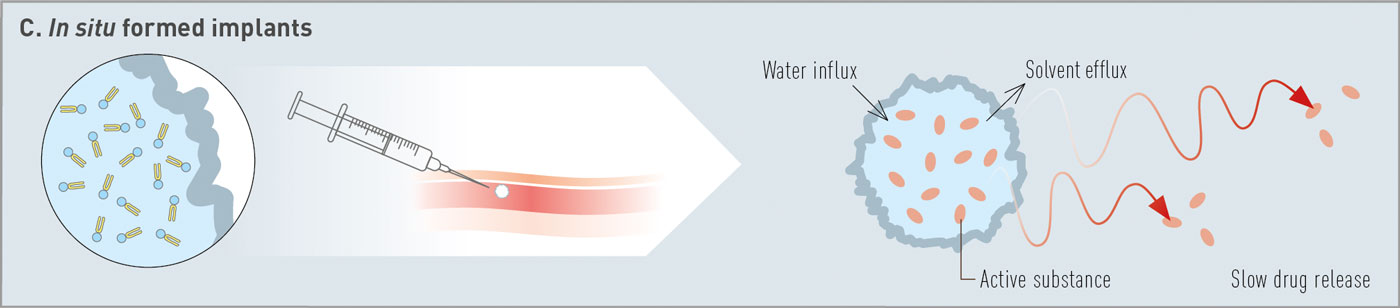
Fig. 1: Overview of categories of phospholipid-based depot technologies: (A) drug suspension, (B) multivesicular liposomes, (C) in situ formed depots at injection site.
Compared to polymer depots, phospholipid depots exhibit favorable degradation profiles related to the formation of less acidic products. All this together makes phospholipids highly interesting excipients for depot injectables.
Phospholipids are nowadays successfully used in numerous approved and marketed depot formulations and are being investigated in ongoing research and development projects using various technologies.
Lipoid offers a wide range of large-scale available natural and synthetic phospholipids of pharmaceutical quality to formulate innovative depot injectables with optimized drug release profiles.